
RNA Functional Analysis
Empower your RNA functional studies with our LNA-enhanced research tools
Discovering new RNA function
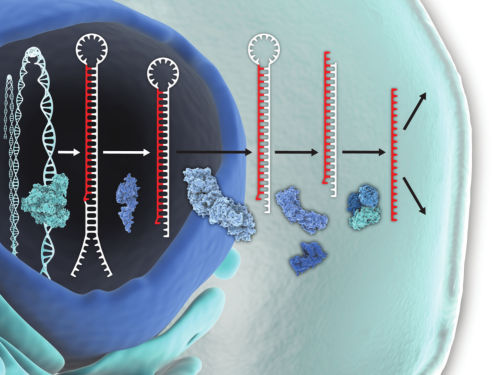
mRNA/lncRNA silencing
LNA GapmeRs are antisense oligonucleotides (ASOs) 15–16 nucleotides long that provide highly effective mRNA/lncRNA silencing both in vitro and in vivo. These oligos contain a central stretch (“gap”) of DNA monomers flanked by blocks of LNA-modified nucleotides, which enable increased target affinity regardless of GC content. The central DNA gap activates RNase H cleavage of the target RNA upon binding. Designing LNA GapmeRs can be complicated, but our in-house experts can optimize designs for high potency and success.
Fully phosphorothioated backbones provide exceptional resistance to enzymatic degradation, and because they are single stranded, LNA GapmeRs allow strand-specific knockdown of RNAs and minimize off-target effects, due to the lack of a passenger strand. Since LNA GapmeRs act independently of the RNA induced silencing complex (RISC), there are no issues with saturation of RISC. The mRNA knockdown efficacy rivals that of siRNA, so LNA GapmeRs are an excellent alternative for researchers looking for a method that works independently of RISC with no miRNA-like off-target effects.
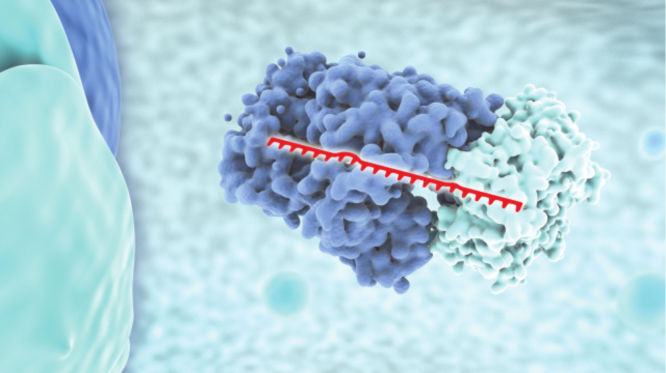
miRNA mimicry
miRNA mimics simulate the natural functions of endogenous miRNAs and are primarily used in gain-of-function studies. Introducing an miRNA mimic into cells increases the proportion of RISCs containing the guide strand miRNA, enabling assessment of the phenotypic consequences of increased activity and discovery of new miRNA functions. Our miRCURY LNA miRNA Mimics have an innovative design that includes two short, LNA-enhanced complimentary strands that prevent any miRNA-like activity associated with the passenger strands, ensuring that phenotypes observed using these mimics are due to increased activity of the mimicked miRNA only.
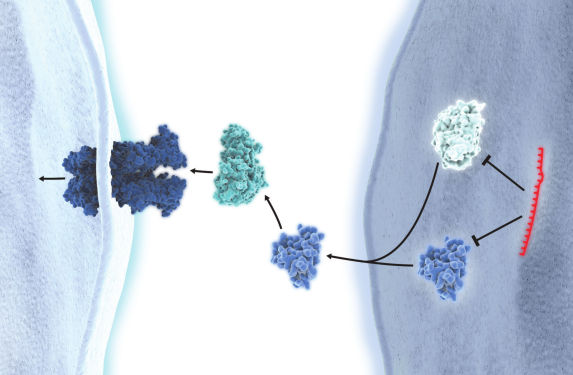
miRNA inhibition
miRCURY LNA miRNA Inhibitors are ASOs with perfect sequence complementary to their miRNA targets. When introduced into cell cultures or animal models, they sequester the target miRNA in highly stable heteroduplexes, effectively preventing the miRNA from hybridizing with its normal cellular interaction partners. This specific suppression of miRNA activity can help elucidate the role of miRNAs in cellular processes and pathological pathways or identify and validate miRNA targets in vitro and in vivo.
All of our miRCURY LNA miRNA Inhibitors were developed with optimal LNA length, sequence and positioning and are also Tm-normalized. This ensures high uniform potency towards all miRNAs, regardless of GC content, along with excellent specificity and biological stability. As an added benefit, these inhibitors are easily taken up without the need for cholesterol conjugation. Plus, because the LNA bases are distributed throughout the entire length, the LNA inhibitor/RNA duplexes are not recognized as substrates for RNase H. This reduces off-target effects on unrelated, longer RNAs that share the same target sequence.
Identification of important miRNA targets
miRNAs are typically involved in regulating a large number of genes, so the phenotype observed due to changes in miRNA activity is actually the combined effect of deregulating several targets. Very often, a few or only just one target is the main contributor to the phenotype. Identifying this target is important to understanding miRNA function. A target site blocker (TSB) – an ASO that specifically prevents interaction of a miRNA with one of its RNA targets – allows researchers to assess the effects of a miRNA on a single target. The TSB masks the miRNA target site in the RNA target of interest, providing specific de-repression of a single intended target only, and enabling simple phenotypic interpretation.
Our miRCURY LNA Target Site Blockers are available with high purity and PK/PD properties optimized for high potency in vivo studies. LNA enhancement stabilizes the TSBs, allowing effective distribution in animal models, so you can make discoveries that might not be possible in cell culture. TSBs are also easily taken up without the need for cholesterol conjugation.
Tools for high-impact results
QIAGEN’s broad offering of LNA research tools ensures high potency and high specificity analyses, for full elucidation of RNA function – both in vitro and in vivo. Available in a variety of synthesis scales with various custom modifications, you can be sure to find the tool to meet your particular research needs.
miRCURY LNA miRNA Target Site Blockers
- Mask specific miRNA target sites or ribonucleoprotein binding sites on mRNA and lncRNA
- Identify miRNA targets in vitro and in vivo
miRCURY LNA miRNA Inhibitors
- Potent miRNA inhibition
- Sequester miRNA in stable inactive complex and prevent loading into RISC
- Effective in vitro and in vivo
miRCURY LNA miRNA Mimics
- Unique triple RNA strand design ensures specific miRNA mimicry
- LNA-enhanced segmented passenger strand
- Only unmodified active miRNA guide strand is loaded into RISC
Antisense LNA GapmeRs
- Potent silencing of any of mRNA or lncRNA
- RNase H dependent degradation of complementary RNA targets
- Effective in vitro and in vivo
Resources & publications
Ready to learn more? Here is a selection of publications, webinars and other resources that demonstrate the power of QIAGEN's LNA-enhanced functional analysis tools.
Resources
Download our functional analysis brochure to learn more about our functional analysis portfolio.
Watch a webinar: "Competing RNAs analyzed with LNA-enhanced functional tools: A non-coding function of TYRP1 mRNA promotes melanoma growth" by Dr. Marie-Dominique Galibert, Rennes University.
Application stories
Prof. Thomas Thum and Dr. Janika Viereck work at the Institute of Molecular and Translational Therapeutic Strategies, Hannover Medical School, Germany. They recently published a Science Translational Medicine paper on the lncRNA Chast and its involvement in cardiac remodelling. They have been using Antisense LNA GapmeRs in vivo to silence Chast and reverse cardiac disease. Read more
Prof. Jean-Christophe Marine and Dr. Eleonora Leucci work at the Laboratory for Molecular Cancer Biology, K.U. Leuven in Belgium. They recently published a Nature paper showing that melanoma cancer cells are addicted to the lncRNA SAMSSON. They have been using Antisense LNA GapmeRs to silence the lncRNA SAMSSON and suppress tumor growth. Read more
Selected publications:
miRCURY LNA in vivo Power Target Site Blockers
- Messina, A. et al. (2016) A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nature Neuroscience, 19, 835. (Link)
- Lippi, G. et al. (2016) MicroRNA-101 regulates multiple developmental programs to constrain excitation in adult neural networks. Neuron, 92:6, 1337–1351. (Link)
- Gilot, D. et al. (2017) A non-coding function of TYRP1 mRNA promotes melanoma growth. Nature Cell Biology, 19, 1348. (Link)
- Sonneville, F. et al. (2017) MicroRNA-9 downregulates the ANO1 chloride channel and contributes to cystic fibrosis lung pathology. Nature communications, 8:1, 710. (Link)
miRCURY LNA in vivo miRNA inhibitors
- Kornfeld, J.-W. et al. (2013) Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature, 494, 111. (Link)
- Boon, R. A. et al. (2013) MicroRNA-34a regulates cardiac ageing and function. Nature, 495, 107. (Link)
- Sene, A. et al. (2013) Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell metabolism, 17:4, 549–561. (Link)
- Son, D. J. et al. (2013) The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nature communications, 4, 3000. (Link)
- Seeger, T. et al. (2014) Long-term inhibition of miR-21 leads to reduction of obesity in db/db mice. Obesity, 22:11, 2352–2360. (Link)
- Sassi, Y. et al. (2017) Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nature communications, 8:1, 1614. (Link)
miRCURY LNA GapmeRs
- Xing, Z. et al. (2014) lncRNA directs cooperative epigenetic regulation downstream of chemokine signals. Cell, 159:5, 1110–1125. (Link)
- Viereck, J. et al. (2016) Long noncoding RNA Chast promotes cardiac remodeling. Science Translational Medicine, 8:326, 326ra22. (Link)
- Adriaens, C. et al. (2016) p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nature Medicine, 22, 861. (Link)
- Lin, A. et al. (2017) The LINK-A lncRNA interacts with PtdIns(3,4,5)P(3) to hyperactivate AKT and confer resistance to AKT inhibitors. Nature cell biology, 19:3, 238–251. (Link)


